Thank you for using rssforward.com! This service has been made possible by all our customers. In order to provide a sustainable, best of the breed RSS to Email experience, we've chosen to keep this as a paid subscription service. If you are satisfied with your free trial, please sign-up today. Subscriptions without a plan would soon be removed. Thank you!
MAGIC through two MILLENNIA
WAVE-PARTICLE DUALITY
QUOTABLE QUOTES
1. Common sense is the deposit of prejudice laid down in the mind before the age of 18. A. Einstein
2. God is a mathematician of a very high order and He used very advanced mathematics in constructing the Universe. P.A.M. Dirac
3. If you are not confused by Quantum Physics then you haven't really understood it. N.Bohr
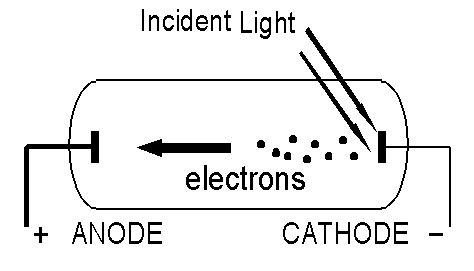
What is the Photo-Electric Effect?
In the Photo-Electric effect, a metal is illuminated with light. Under certain circumstances, electrons are emitted from the illuminated surface. We can vary the intensity of the light and its frequency (its colour).Expectations of Classical Physics.
These expectations are based on the belief that light is an electromagnetic wave; if we increase the intensity of the light this is equivalent to increasing the amplitude of the oscillating electric field of which the light wave is composed. Since the energy of the light beam is spread uniformly over the beam, it is transferred continuously to the electrons, which require a certain minimum of energy to escape the attractive forces of the metal. In the following, "The maximum energy of the electrons" means "The energy of the most energetic electrons".
- a. Whatever the frequency of the incident light, electrons will be emitted from the metal surface as long as the light carries enough energy - i.e. if it is sufficiently intense.b. If the intensity of the light is increased the maximum energy of the electrons should also increase. c. There may be a time delay between the switching on of the light and the appearance of the first electrons; the lower the light intensity, the longer will be this time delay.
Experimental Observations.
- a. Not one electron is emitted if the frequency of the light is below a certain value (called the "threshold frequency", which is dependent on the type of the metal). Above this threshold frequency, the maximum energy of the emitted electrons increases in direct proportion to the frequency of the light.b. The maximum energy of the emitted electrons is independent of the intensity of the light. c. No matter how weak the light, as long as its frequency is above the threshold frequency, the emission of electrons starts IMMEDIATELY the light is switched on.
Einstein's Explanation.
- a. Light comes in LUMPS which we call photons.b. The energy of each photon is directly proportional to the frequency of the light. c. The interaction between a photon and an electron in the metal is a unique, elemental act, in which the photon can give up some, or all of its energy to the electron, which then might have enough energy to escape from the metal.
Einstein's conjecture that the energy of a photon is proportional to its frequency can be written Ephoton = h f ; here h is Planck's constant.
Click here to return to top of document
Particles are also Waves.
The great success of Einstein's theory for the photoelectric effect stimulate de Broglie to postulate in his Ph.D. thesis at the University of Paris, that particles might exhibit wave-like properties. Starting from Einstein's relation, Ephoton = h f and using the relation given by Maxwell's equations for the momentum of a light wave,Elight = pc (where p is the momentum of the light, and c is the speed of light), de Broglie derived the expression p =h/In 1927, three years after de Broglie's proposal, Davisson and Germer, working at Bell Labs in the US, and, independently G.P. Thomson working at Cambridge University, observed interference patterns in the scattering of electrons. The "wavelength" of the electrons, calculated from the observed interference patterns, agreed exactly with de Broglie's formula.
--
Source: http://basistik.blogspot.com/2011/06/wave-particle-duality.html
~
Manage subscription | Powered by rssforward.com



0 comments:
Post a Comment